Features

e-QUALi®'s powerful features
Find out more about the exceptional features that make e-QUALi® the essential solution for managing qualifications and validations in industry.
From digital testing to electronic signatures, explore how e-QUALi® can revolutionize your approach to quality.
e-QUALi® features in detail
Find out how e-QUALi® simplifies every stage in the management of qualifications and validations. From preparation to execution and analysis, our digital solution meets your needs.
Prepare the URS and the various documentary versions in a single space.
Produce and validate your FAT, SAT, QI, QO with a single tool.
Keep your history at your fingertips and optimize your next interventions.
e-QUALi® lets you carry out tests directly on your device. PCs, tablets, smartphones, no need to print.
The audit trail is an essential part of the 21 CFR principles. e-QUALi® records and time-stamps each user's actions (additions, modifications and deletions).
e-QUALi® works with a PDF editor. This allows you to directly import your existing files and edit them (writing, highlighting, underlining, etc.) directly on our platform. Each action is of course recorded in our audit trail.
e-QUALi® enables you to create and manage deviations in every phase of your projects. These deviations can be linked to one or more tests, depending on your preferences.
Sign your documents electronically directly on e-QUALi®.
Our cloud solution instantly updates your projects and allows all stakeholders to track progress.
Real-time updating without rewriting means that everyone involved can work asynchronously, especially for document review.
e-QUALi® was developed by engineers specializing in industrial commissioning in the pharmaceutical sector.
Quickly create test sheets and professional documents with our intuitive interface.
Import, track and approve your documents with ease.
Ready to Start Your Transformation?
Contact our team today to find out more about e-QUALi®, obtain further information or schedule a personalized demonstration.
We’re here to answer all your questions and help you get started on your journey towards more efficient qualification and validation management.
Prepare
Prepare your documents easily
With e-QUALi®, preparation of the URS (User Requirement Specification) and the various document versions is simplified. Our solution gives you a single place to organize and manage all your documents.
- Create and manage your documents with ease.
- Centralize access to URS and document versions.
- Facilitate collaboration between team members.


Run
Test with confidence
e-QUALi® lets you carry out and validate your FAT (Factory Acceptance Tests), SAT (Site Acceptance Tests), IQ (Installation Qualification) and OQ (Operational Qualification) with a single tool. Save time and ensure that your tests are carried out correctly.
- Run your tests directly on the platform.
- Ensure regulatory compliance.
- Simplify test documentation management.
Analyze
Analyze your data in depth
With e-QUALi®, the history of all your actions is just a click away. Analyze your data and identify optimization opportunities for future operations.
- View stock history in real time.
- Identify trends and potential improvements.
- Make informed decisions about your qualification and validation projects.


Performing your tests
Digital Testing
e-QUALi® lets you carry out your tests directly on your device, be it a PC, tablet or smartphone. Eliminate the need to print numerous documents, save time and reduce your carbon footprint.
- Perform tests using electronic devices.
- Reduce document printing costs.
- Help protect the environment by reducing paper waste.
Audit Trail
Follow Every Action with the Audit Trail
The audit trail is an essential part of the 21 CFR principles. e-QUALi® records and time-stamps each user’s actions (additions, modifications and deletions). A macro version of the test is just a click away.
- Guarantee traceability of all actions.
- Meet the regulatory requirements of 21 CFR.
- Quick access to summary versions of tests.


Edit your PDFs easily
Edit your PDFs easily
e-QUALi® features an integrated PDF editor. Import your existing files and perform edits such as writing, highlighting and underlining directly on our platform. Every action is recorded in our audit trail.
- Easily import and edit PDF files.
- Collaborate with colleagues on PDF documents.
- Keep a complete record of all file modifications.
Deviation management
Manage your detour efficiently
e-QUALi® lets you create and manage deviations in every phase of your projects. These deviations can be linked to one or more tests, depending on your preferences. Make sure your projects progress smoothly.
- Create deviations and track their resolution.
- Customize deviation management to suit your needs.
- Reduce delays and interruptions in your qualification and validation projects.


Electronic Signature
Sign your documents electronically
With e-QUALi®, you can electronically sign your documents directly on the platform. Save time and ensure the validity and integrity of your electronic signatures.
- Perform electronic signatures in compliance with regulatory standards.
- Reduce the need for manual signatures on paper documents.
- Increase the security and authenticity of your documents.
Real-time tracking
Track progress in real time
Our cloud solution instantly updates your qualification and validation projects. All stakeholders can monitor progress in real time.
- Keep all stakeholders informed in real time.
- Reduce communication delays.
- Facilitate remote collaboration on projects.
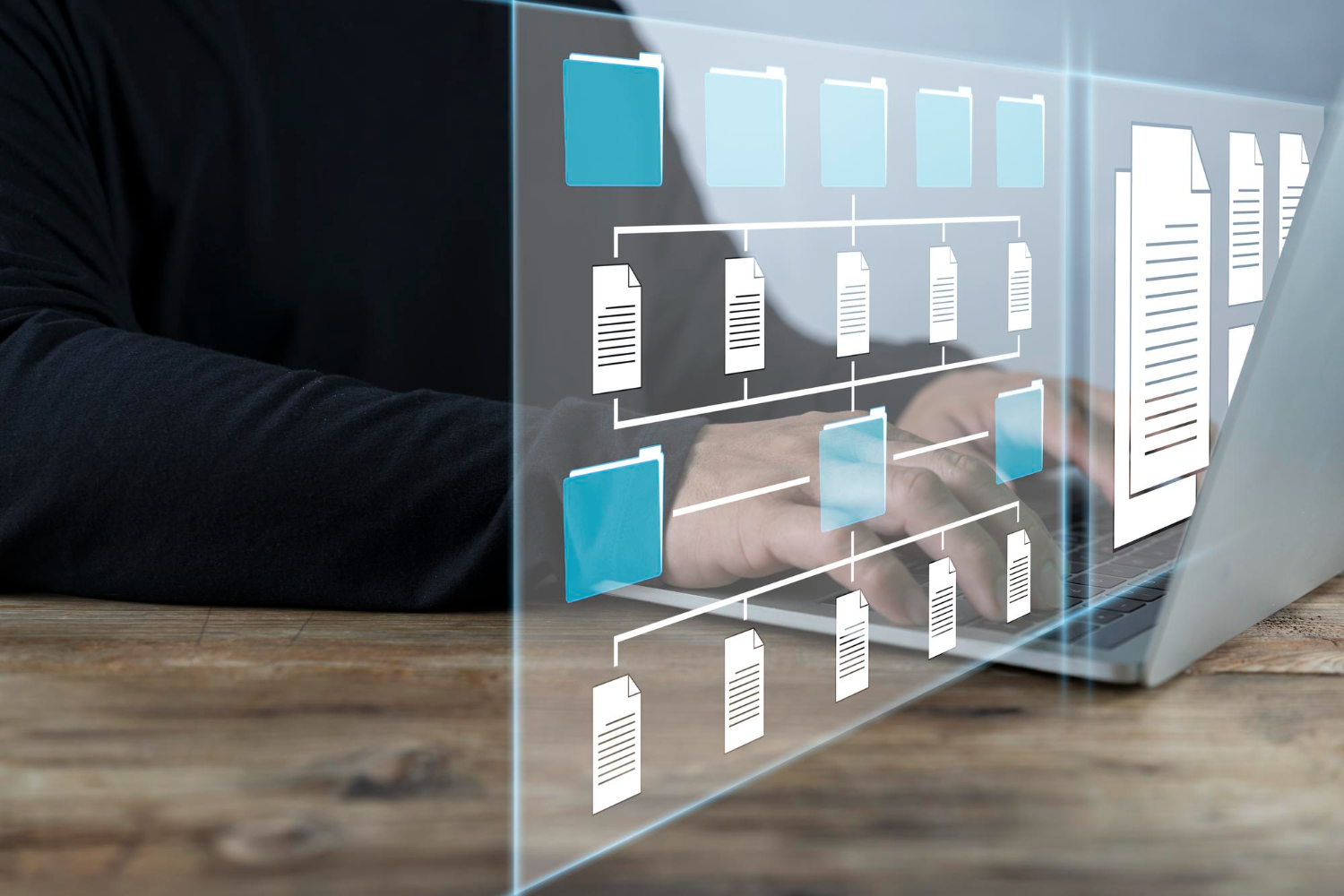

Asynchronous working
Work in Asynchronous Mode
Real-time updating without rewriting means that everyone involved can work asynchronously, especially for document review. Gain in flexibility and efficiency.
- Eliminate the need for real-time proofreading meetings.
- Let your team work to their own schedule.
- Reduce document review and approval times.
Proven User Experience
A solution designed by experts
e-QUALi® was developed by engineers specializing in industrial commissioning in the pharmaceutical sector. Benefit from the expertise of industry professionals.
- Benefit from a solution tailored to the specific needs of the pharmaceutical industry.
- Benefit from expert support and advice on qualification and validation.
- Join thousands of satisfied e-QUALi® users.

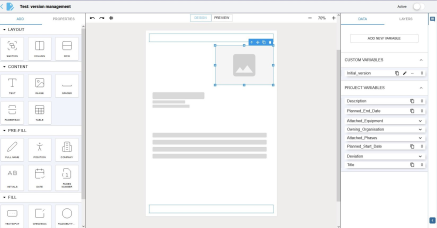
Powerful Template Generator
Template Creation Module
Our powerful template generation engine lets you quickly and easily create test sheets or professional documents for your projects. With our intuitive interface and pre-designed templates, you don’t need any technical expertise. Create new templates quickly by copying an existing one.
Manage your documents with ease
Document Management System
Create your documents outside e-QUALi® and import them into your project. Track all versions and modifications of your documents. Go through the entire acceptance process: review, approval and electronic signature. Anytime, anywhere, you have access to all your document activity. No more errors: you’ll always be working on the latest approved version.

Ready to Start Your Transformation?
Contact our team today to find out more about e-QUALi®, obtain further information or schedule a personalized demonstration.
We’re here to answer all your questions and help you get started on your journey towards more efficient qualification and validation management.

